A lot of interesting case reports get published in human medical and veterinary journals, but I always take case reports with a grain of salt. It’s not that I don’t trust the validity of the report, but there are those who may over-react to a single case. A publication about a single case typically signifies that it’s an oddball occurrence, and probably has limited broader significance. However, sometimes case reports can be an early indicator of something emerging, or something that we’ve missed in the past. Sorting out those possibilities can be challenge.
With that in mind, I want to talk about a recent case report (Bradbury et al. 2026) published in the American Journal of Tropical Medicine and Hygiene, which describes a person in Australia who had an intestinal infection with Ancylostoma caninum, the canine hookworm.
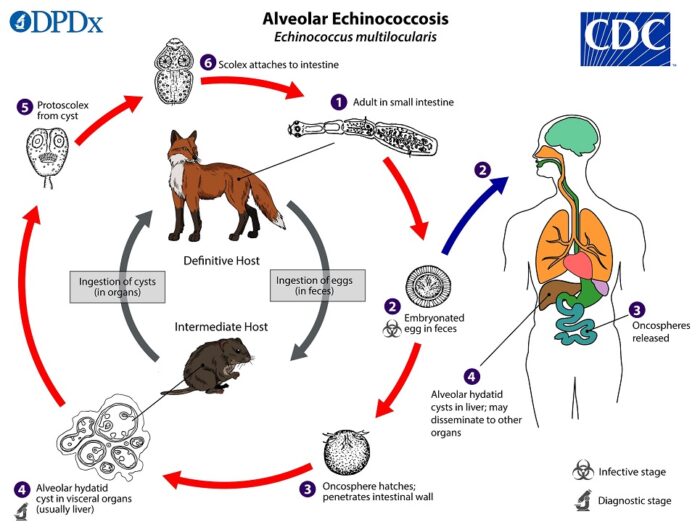
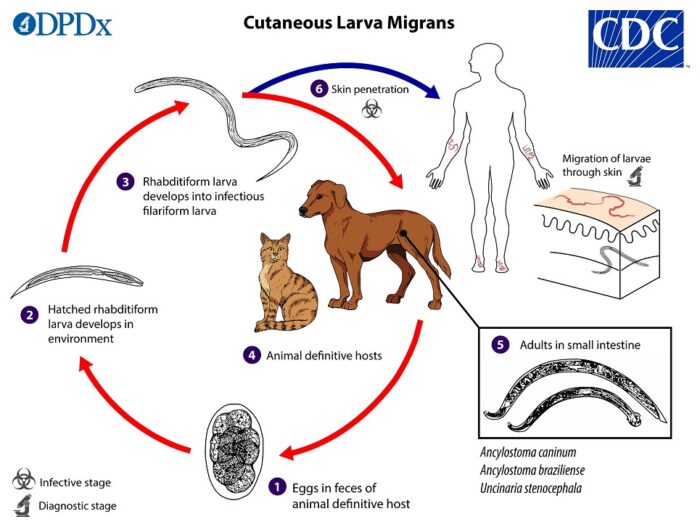
Ancylostoma caninum is a common hookworm in many areas that can cause disease in dogs (especially puppies) but is more often shed in the feces of healthy dogs with no clinical signs at all. Adult worms live in the dog’s small intestine, where they produce eggs that are shed in dog’s feces. The eggs then hatch in the environment, go through a few larval stages, and if then ingested by another dog, develop into adult worms in the intestine and the cycle begins again.
Human infections with this parasite are not unusual in some regions either, but in people the infection takes the form of cutaneous larva migrans, where the larvae from the environment penetrate the skin and cause intensely itchy lesions while they migrate around, fruitlessly looking for their natural site of infection (dog intestines).
The normal life cycle of A. caninum in dogs, with spillover infection causing cutaneous larva migrans in people, is shown below:
The larvae shouldn’t be able to develop into adult worms in human intestines, but biology is full of exceptions, and rare cases do occur (like the one I wrote about back in 2019). Unusual zoonotic infections / forms of infection are more likely to occur in people with weakened immune systems, but it is noteworthy that the case from this latest report was in a 35-year-old man with a normal immune system. The patient had a recurrent history (over years) of hemorrhoids and bleeding from the rectum, so he had a colonoscopy. Along with some intestinal polyps, they found several areas where the intestinal lining had aphthous erosions (these are similar in appearance to canker sores some people get in their mouths). They also found a live worm within one of these eroded regions, and the worm was identified as A. caninum based on its appearance and DNA sequencing. When biopsies of the erosions were evaluated, they showed eosinophilic inflammation, consistent with lesions caused by a parasitic infection.
The patient was treated with an antiparasitic (dewormer). They didn’t test his feces for parasites or eggs, because it’s assumed that in these aberrant infections in people the worms don’t develop past the subadult stage, so they don’t produce eggs (but it would have been nice to confirm that). That’s good because it means the person wouldn’t be able to spread the infection, but also makes it harder to detect the infection in the first place. That raises the question about whether intestinal infections like this could be more common that we think.
It was suspected that the hookworm was more of an incidental finding, as the rectal bleeding was more likely associated with the hemorrhoids. However, it’s possible that the worm could have been causing some disease, as anything that triggers inflammation and erosion in the gut is a potential concern.
Another interesting consideration is that the worm was found in the colon. In dogs, this parasite likes to live in the small intestine (which is much farther upstream). Hookworms are usually only found in the colon when a dog has a severe infestation. This raises the question of whether the patient might have had a more extensive infection than was not detected, since only the colon was evaluated.
What does this change? Not much. It’s an interesting case, and a good reminder of the risks from zoonotic pathogens and the potential for underestimating the amount of animal-to-human and human-to-animal transmission of various organisms. The take home messages are the same though:
- don’t eat poop
- avoid environments with heavy fecal contamination – and wash your hands!
- pick up animal feces and dispose of them appropriately, so that any parasites in them don’t get a chance to find their next host (be it human or canine or other).
More information about hookworms in animals can be found on the Worms & Germs Resources – Pets page.